Our Vision
Our Technologies
PHOTO-CROSSLINKED, PRE-FABRICATED IMPLANTS ≤2 µg/day for 6 Mo.
EyeLief® & EyeLief-SD™
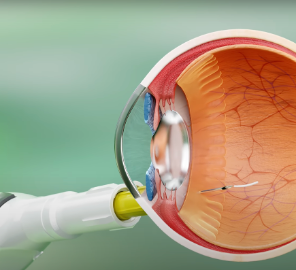
Pre-fabricated IVT implants photo-crosslinked using UV light, administered in the clinic via narrow gauge needles, designed to deliver a range of therapeutic modalities, including Biologics, Peptides, Large and Small Molecules.
IN SITU PHOTO-CROSSLINKED ≥2 µg/day for 6-12 Mo.
OcuLief®
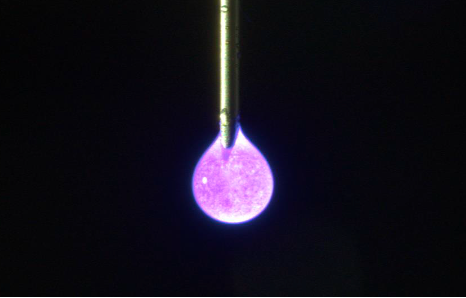
In situ fabricated IVT implants, photo-crosslinked using visible light, administered in the clinic via narrow gauge needles and a bespoke applicator, delivering high doses as a liquid that cures into a solid implant for controlled, sustained release of a range of therapeutic modalities, including Biologics, Peptides, Large and Small Molecules.
Our Strategies
Solving the No. 1 Unmet Need
Reducing the frequent injection burden of anti VEGF’s, Biologics, Peptides, Large and Small Molecules
Breakthrough in Biologic Delivery
Photo-crosslinked biodegradable hydrogel: protects and preserves Biologics ensuring customizable tunable release with proven tolerability (avoids common cause of inflammation)
1st In-Clinic Technology
Potential to deliver 0.05 µg/day to 20 µg/day for up to 12 months

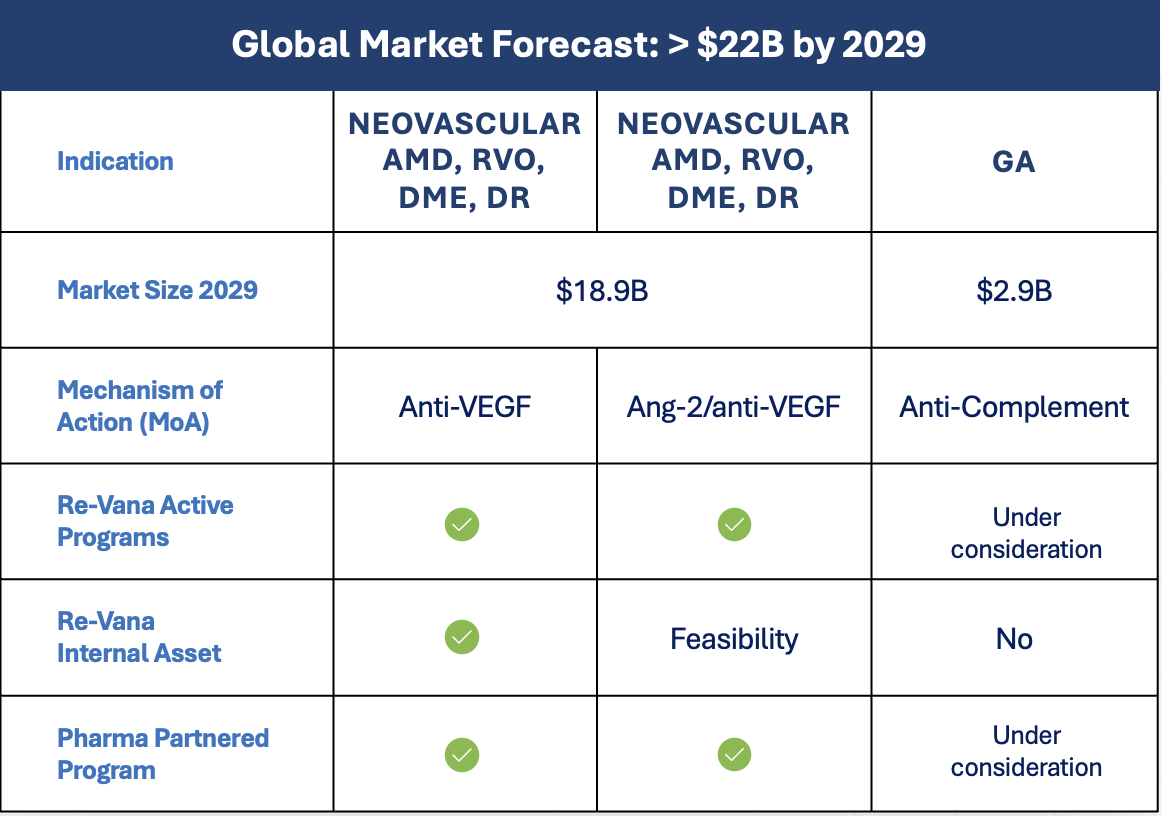
Strategic Collaborations
Active partnerships with leading global pharmaceutical companies including Boehringer Ingelheim
Partnerships and Collaborations
Re-Vana is working with several global pharmaceutical companies in conducting preformulation and preclinical feasibility studies.