OcuLief®
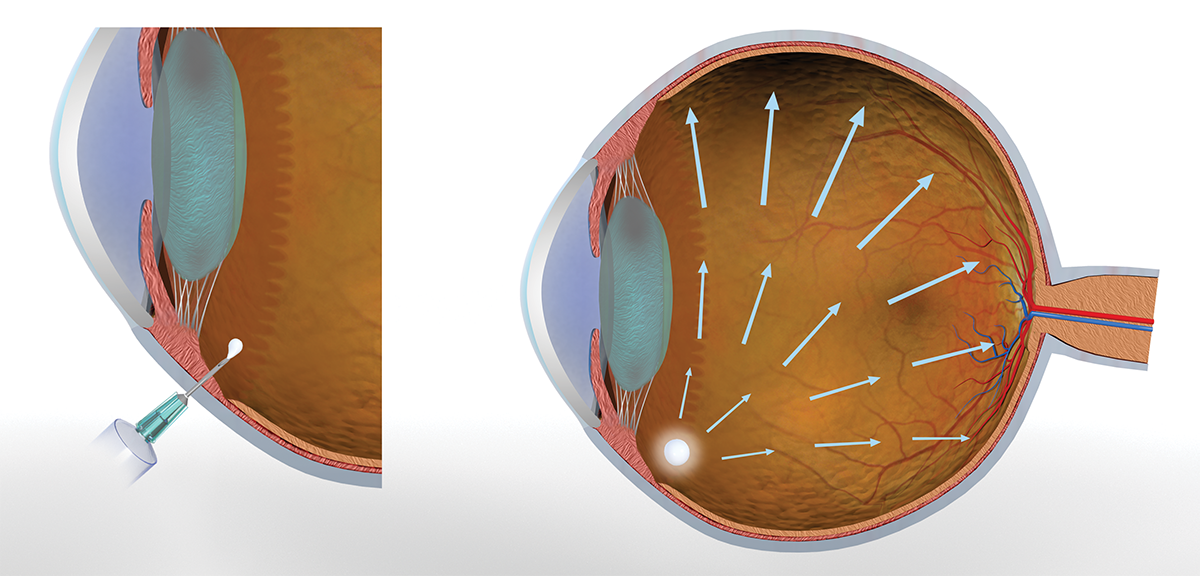
OcuLief® is an in situ depot forming injectable gel-based implant.
It is composed of gel made from polymeric materials that upon exposure of visible light results in a photocrosslinked implant in the eye. The gels are injected in the eye using narrow gauge needles. This photocrosslinked biodegradable implant is designed for less potent drug molecules that require higher release rates and also provides sustained biologic delivery for 6+ months.
Advantages of OcuLief®
- Gel-based system injected into the eye and photo crosslinked in situ
- Injected in the eye using narrow gauge needles – office based delivery
- Novel applicator with a built-in visible light source
- Designed for less potent molecules that require higher release rates
