EyeLief®– EyeLief-SD™
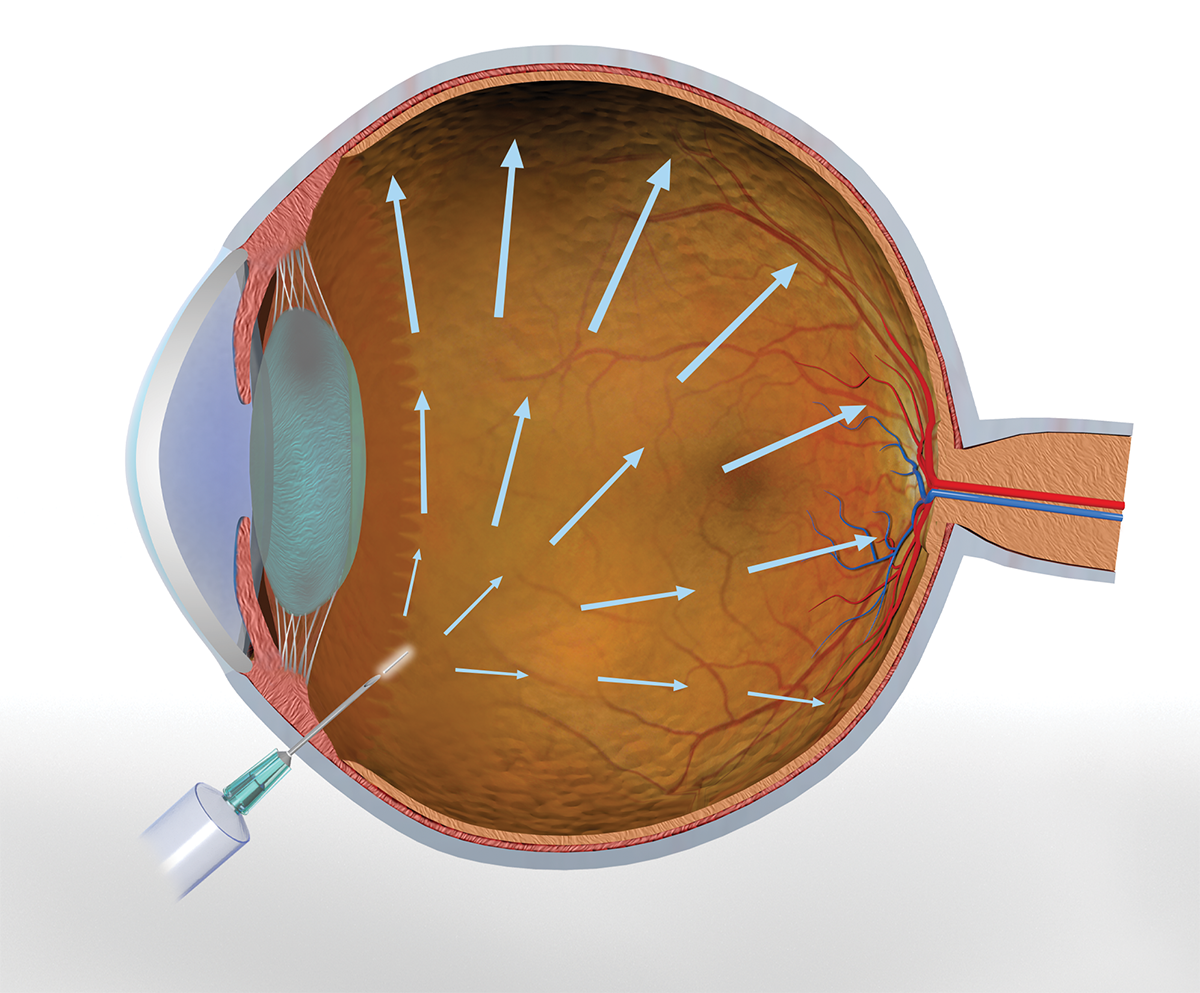
EyeLief® is a preformed implant, composed of our photocrosslinked polymeric matrix that is designed to be injected into the eye through narrow gauge needles.
This implant is the first biodegradable implant that can achieve significant high loading of both small molecule and biologic drugs. In-vitro and animal studies have demonstrated sustained drug delivery for 6+ months and safety of this implant.
In preclinical studies the EyeLief implant demonstrated excellent tolerability following intravitreal and intracameral administration. In a six month safety and PK study there was no evidence of irritation or significant inflammatory response following intravitreal injection of the implant. Re-Vana is currently conducting additional preclinical tests and studies to enable the company to move to future human clinical trials.
EyeLief-SD (Super Dense) provides higher release rates and drug loading, thus broadening the wide range of biologics and small molecule therapeutics capable of 6+ months sustained release.
Advantages of EyeLief®-EyeLief-SD™
- Injected in the eye using narrow gauge needles – office based delivery
- Provides hydrophilic environment – ideal for maintaining protein/biologic stability
- Provides improved protein/biologic stability
- Sustained release of biologics and small molecules of 6+ months
- High loading of biologics and small molecules
- Customized release profiles address a wide range of clinical needs
