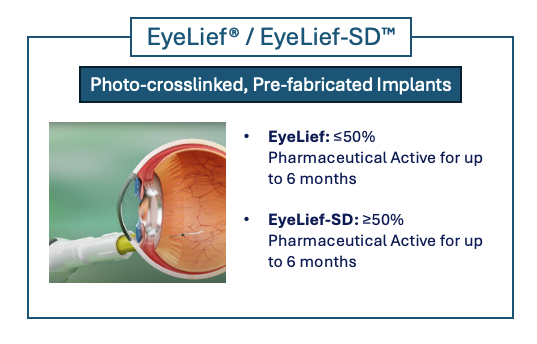
EyeLief®– EyeLief-SD™
EyeLief®/EyeLief-SD™ implant technology are patented photo-crosslinked, prefabricated implants which can be administered using a narrow-gauge needle.
Re-Vana implants can be customized for a variety of release profiles. The preformed implant enables sustained release of the drug and is suitable for biologics, peptides, large and small molecules.
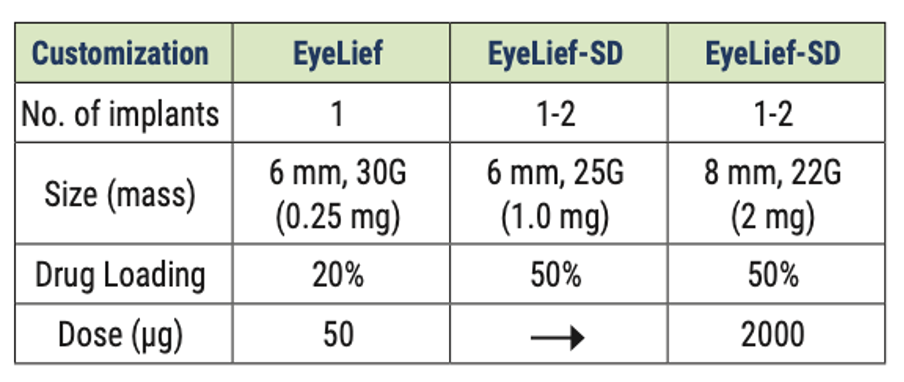
The two technologies enable sustained drug release; each tailored to specific therapeutic requirements. Re-Vana implants can carry up to 50% of their weight in active pharmaceutical ingredients (API) and are engineered to release potent drugs steadily over a period of 3 to 12 months.
Factors influencing the rate and duration of drug release from Re-Vana implants include dimension, number of implants, choice of macromer, drug loading and crosslink density.
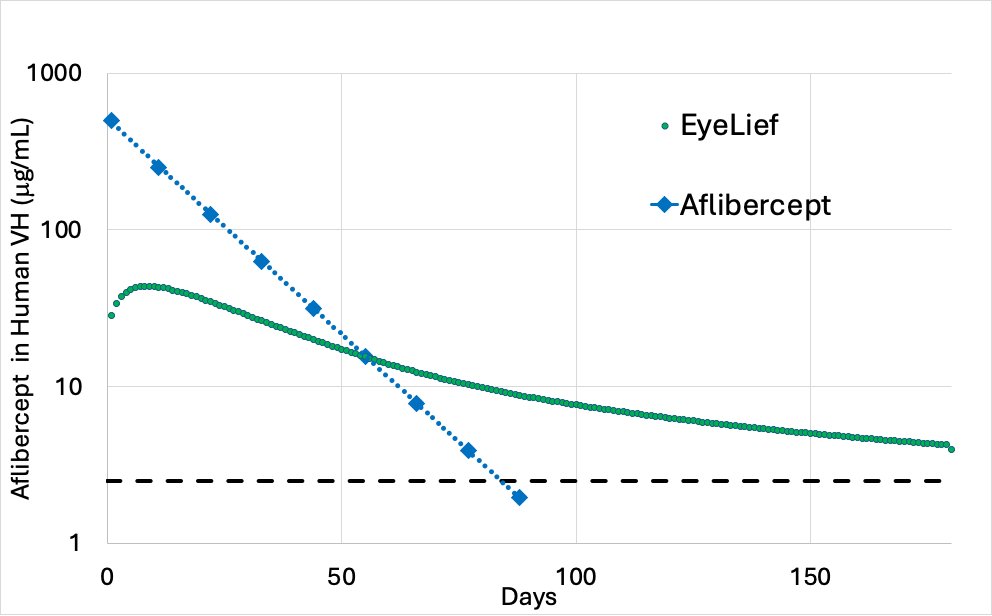
Reducing injection frequency for AMD and DR patients remains a key priority across the industry however this requires an optimal PK profile of which there is no current product offering
- In-clinic IVT injections of aflibercept can achieve approximately 2-3 months clinically relevant aflibercept
- Re-Vana’s approach to reducing injection frequency leverages EyeLief® aflibercept that maintains clinically relevant concentrations of aflibercept for up to 6-months; the EyeLief® implant is designed to fully biodegrade within 12 months, allowing for repeat treatments to manage this chronic disease.